Regenerative therapies with stem cells
Legal framework for regenerative medicine
Regenerative medicine has been rapidly developing and drawing increasing attention as an innovative treatment option. However, without ensuring safety and efficacy, the risk to patients increases, and the sound development of medical technologies may be hindered.
Accordingly, proper implementation of regenerative medicine is governed by two key laws in Japan: the Pharmaceuticals and Medical Devices Act (PMD Act) and the Act on the Safety of Regenerative Medicine.
Regenerative medicine in Japan is provided under two main categories: 'regenerative medical products,' which are offered under public health insurance and regulated by the Pharmaceuticals and Medical Devices Act (PMD Act), and 'Specific Processed Cells', which are offered as part of private (uninsured) medical care and regulated under the Act on the Safety of Regenerative Medicine.
TOPs cells fall under the category of 'Specific Processed Cells' as defined by the Act on the Safety of Regenerative Medicine.
Act on the Promotion of Comprehensive Measures for the Safe and Prompt Provision of Regenerative Medicine to the Public

Manufacture
and marketing
Pharmaceuticals and Medical Devices Act
(PMD Act): Revised in 2014.
| Purpose |
|
|---|---|
| Content |
|
Regenerative
Medical Products
| Definition |
|
|---|---|
| Features |
|

Uninsured
medical care
Clinical
research
Act on the Safety of Regenerative
Medicine: Enforced in 2014.
| Purpose |
|
|---|---|
| Content |
|
Specific
Processed Cells
| Definition |
|
|---|---|
| Features |
|
Risk Classification of Regenerative Medicine Technologies
Under the Act on the Safety of Regenerative Medicine, as illustrated in the flowchart below, regenerative medicine practices are classified into three categories (Classes I, II, and III) based on their potential impact on human life and health. Appropriate regulatory measures are applied to each category.
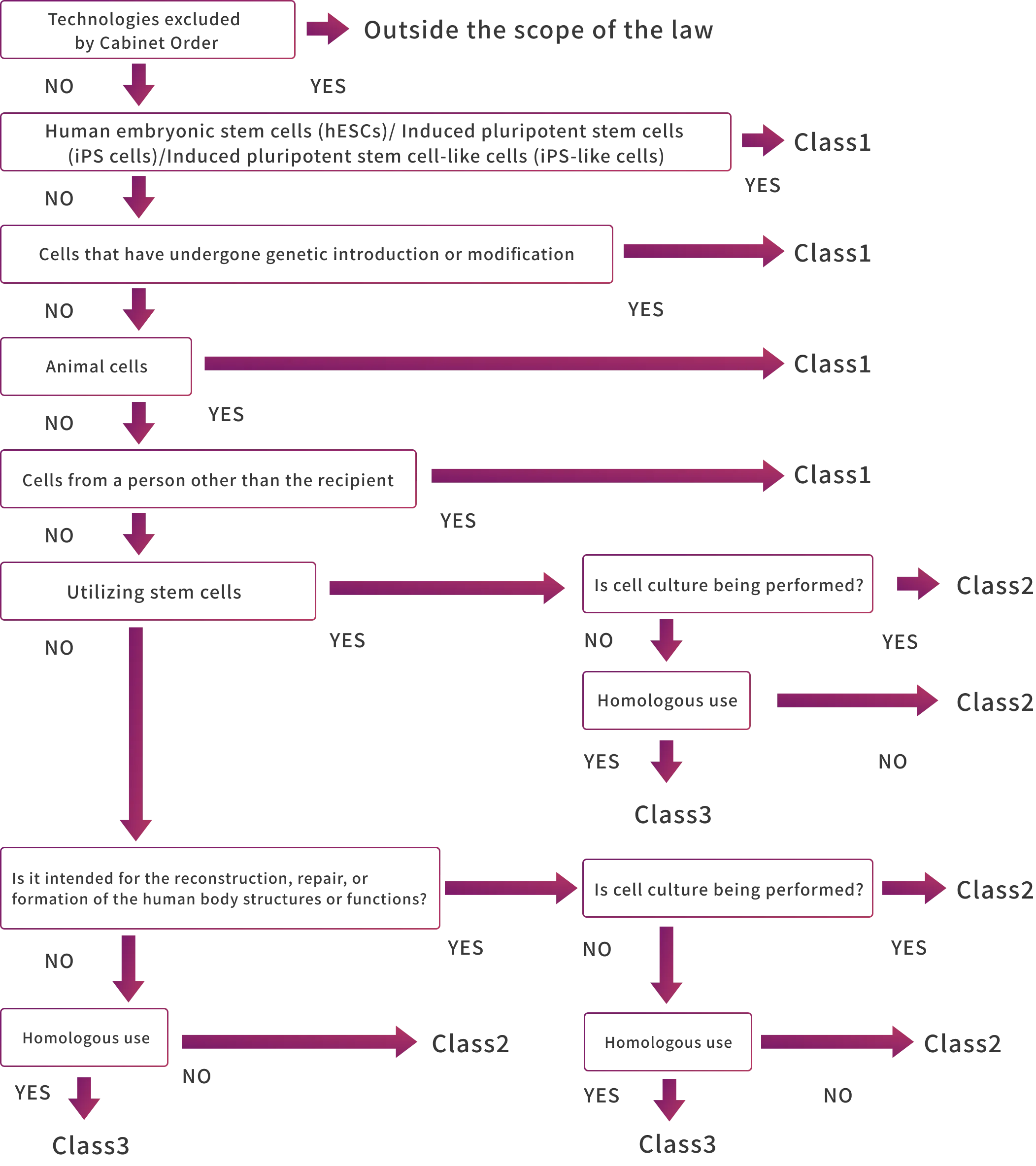
Specific Processed Cells
Under the Act on the Safety of Regenerative Medicine, adipose-derived stem cells (ADSCs) are used to treat various diseases.
A large proportion of regenerative medicine using adipose-derived stem cells falls under Category II regenerative medicine provision plans, accounting for approximately 50% of all treatment plans in this category.
Primary treatment indications
Osteoarthritis of the knee Arthritis
Regeneration of jawbone defects, alveolar bone, and periodontal tissues
Regeneration of ligaments, tendons, and muscles (sports-related injuries)

-
Stem cell therapy
-
What are Stem Cells?
-
Regenerative therapies with stem cells
-
-
Cells
-
Implementing Facility


